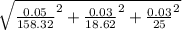Atitration is performed to calculate the concentration of a solution of a monoprotic acid. the buret is filled with a standardized solution of 158.32 ± 0.05 mm naoh. the initial volume is recorded as 0.14 ml. 25.00 ml of the unknown acid solution are pipetted into an erlenmeyer flask and the solution is titrated to a phenolphthalein endpoint. the final buret reading is 18.76 ml. assuming that the error in each volumetric measurement (buret and pipet) is ±0.03 ml, calculate the concentration of the acid (mm) and use propagation of error to estimate its uncertainty.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 08:00, kendrawalraven
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 3


Chemistry, 23.06.2019 15:40, thatlostgirl7740
Which functions of water in living systems would still be possible if water was not polar and did not form hydrogen bonds? check all that apply. climate regulation dissolving ionic compounds for biological reactions providing body support by exerting pressure on cell walls providing body support through buoyancy transport of nutrients within organisms temperature regulation in many organisms
Answers: 3
Do you know the correct answer?
Atitration is performed to calculate the concentration of a solution of a monoprotic acid. the buret...
Questions in other subjects:

English, 24.08.2019 10:10

Physics, 24.08.2019 10:10


English, 24.08.2019 10:10



History, 24.08.2019 10:10


Mathematics, 24.08.2019 10:10

English, 24.08.2019 10:10