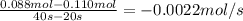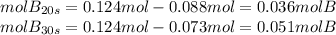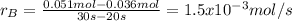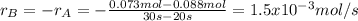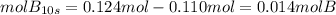Aflask is charged with 0.124 mol of a and allowed to react to from b according to the reaction: a(g) -> b(g). the following drata are obtained for [a] as the reaction proceeds:
time: 0.00 10.0 20.0 30.0 40.0
moles of a: 0.124 0.110 0.088 0.073 0.054
a) what is the average rate of disapperance of a between 10s and 20s?
b) what is the avergae rate of disappearance of a between 20s and 40s?
c) what is the average rate of appearance of b between 20s and 30s?
d) how many moles of b are present at 10s?
e) how many moles of b are present at 30s?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, lucasrandall
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 23:00, 1315055427
Which subshell is represented by the actinides family?
Answers: 1

Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
Do you know the correct answer?
Aflask is charged with 0.124 mol of a and allowed to react to from b according to the reaction: a(g...
Questions in other subjects:

Mathematics, 01.12.2019 15:31

Spanish, 01.12.2019 15:31




Mathematics, 01.12.2019 16:31

Mathematics, 01.12.2019 16:31

Biology, 01.12.2019 16:31

Mathematics, 01.12.2019 16:31