Chemistry, 06.10.2019 05:30, kaylarae1930
Consider the following energy levels of a hypothetical atom: e4 −1.21 × 10−19 j e3 −5.71 × 10−19 j e2 −1.05 × 10−18 j e1 −1.55 × 10−18 j (a) what is the wavelength of the photon needed to excite an electron from e1 to e4? (b) what is the energy (in joules) a photon must have in order to excite an electron from e2 to e3? (c) when an electron drops from the e3 level to the e1 level, the atom is said to undergo emission. calculate the wavelength of the photon emitted in this process.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:20, Richwave17
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 09:00, lrasanaoaksandfurana
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1


Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
Do you know the correct answer?
Consider the following energy levels of a hypothetical atom: e4 −1.21 × 10−19 j e3 −5.71 × 10−19 j e...
Questions in other subjects:


English, 30.08.2019 04:30



Geography, 30.08.2019 04:30

Computers and Technology, 30.08.2019 04:30

Mathematics, 30.08.2019 04:30



History, 30.08.2019 04:30



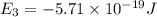


 to
to  .
.
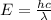
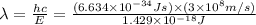
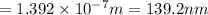
 to
to 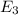 .
.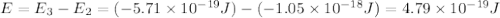
 is the energy a photon to excite an electron from
is the energy a photon to excite an electron from