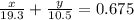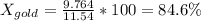Apiece of gold jewelry weighs 11.54 g and has a volume of 0.675 cm3. the jewelry contains only gold (density = 19.3 g/cm3) and silver (density = 10.5 g/cm3). assuming that the total volume of the jewelry is the sum of the volumes of the gold and silver that it contains, calculate the percentage of gold in the jewelry.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1


Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
Do you know the correct answer?
Apiece of gold jewelry weighs 11.54 g and has a volume of 0.675 cm3. the jewelry contains only gold...
Questions in other subjects:



Chemistry, 21.09.2019 00:30

Mathematics, 21.09.2019 00:30

Biology, 21.09.2019 00:30


Mathematics, 21.09.2019 00:30



Mathematics, 21.09.2019 00:30