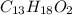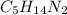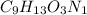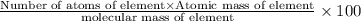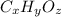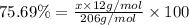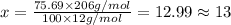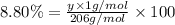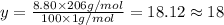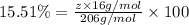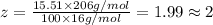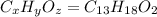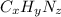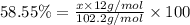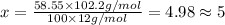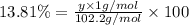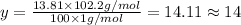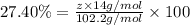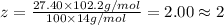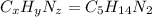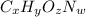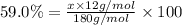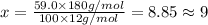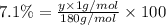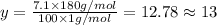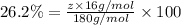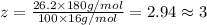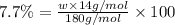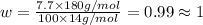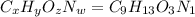Chemistry, 06.10.2019 02:30, xwalker6772
Ibuprofen, a headache remedy, contains 75.69% c, 8.80% h, and 15.51% o by mass and has a molar mass of 206 g/mol. express your answers as chemical formulas separated by a comma. nothing request answer part b cadaverine, a foul-smelling substance produced by the action of bacteria on meat, contains 58.55% c, 13.81% h, and 27.40% n by mass; its molar mass is 102.2 g/mol. express your answers as chemical formulas separated by a comma. nothing request answer part c epinephrine (adrenaline), a hormone secreted into the bloodstream in times of danger or stress, contains 59.0% c, 7.1% h, 26.2% o, and 7.7% n by mass; its molar mass is about 180 amu. express your answers as chemical formulas separated by a comma. nothing

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 00:10, Rubendelarosa1529
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
Do you know the correct answer?
Ibuprofen, a headache remedy, contains 75.69% c, 8.80% h, and 15.51% o by mass and has a molar mass...
Questions in other subjects:

Mathematics, 29.02.2020 02:57

Mathematics, 29.02.2020 02:57




Mathematics, 29.02.2020 02:57