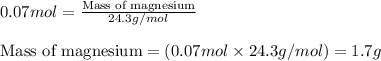Magnesium (used in the manufacture of light alloys) reacts with iron(iii) chloride to form magnesium chloride and iron. 3mg(s) + 2fecl₃(s) → 3mgcl₂(s) + 2fe(s)a mixture of 41.0 g of magnesium ( = 24.31 g/mol) and 175 g of iron(iii) chloride ( = 162.2 g/mol) is allowed to react. identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete. a) limiting reactant is mg: 67g of fecl₃ remains. b) limiting reactant is mg: 134 g fecl₃ remains. c) limiting reactant is mg: 104 g fecl₃ remains. d) limiting reactant is fecl₃: 1.7 g of mg remans. e) limiting reactant is fecl₃: 87.2 g of mg remains.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, gizmo50245
Calculate the mass percent of hydrogen in methyl acetate
Answers: 1

Chemistry, 22.06.2019 12:30, gonzalesalexiaouv1bg
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
Do you know the correct answer?
Magnesium (used in the manufacture of light alloys) reacts with iron(iii) chloride to form magnesium...
Questions in other subjects:

Biology, 28.08.2019 02:00

Mathematics, 28.08.2019 02:00


Arts, 28.08.2019 02:00

Mathematics, 28.08.2019 02:00

Mathematics, 28.08.2019 02:00



Biology, 28.08.2019 02:00


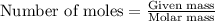 .....(1)
.....(1)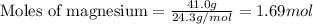
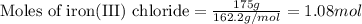
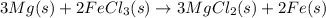
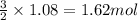 of magnesium
of magnesium