
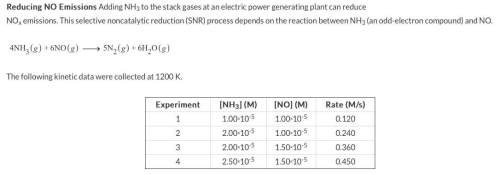
Chemistry, 05.10.2019 16:30, WhatTheFangirl2927
A) what is the rate-law expression for the reaction? do not add multiplication symbols to your answer or states of matter.
rate=
b)what is the value of the rate constant at 1200 k?
-1s-1

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, brasherfamily14
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 06:30, caitybugking
Type the correct answer in the box. spell all words correctly. what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3
Do you know the correct answer?
A) what is the rate-law expression for the reaction? do not add multiplication symbols to your answ...
Questions in other subjects:















