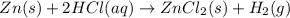When kay added 30 g of hydrochloric acid (hcl) to 65 g of zn, bubbles appeared and a white precipitate, zinc chloride (zncl2) was produced. she found that the mass of the zinc chloride was 93 g. since the law of conservation of matter stated that matter can't be created or destroyed, she was puzzled about the difference in mass. what is the best explanation for the missing 2 g?
a) 2 g of zinc disappeared.
b) 2 g of hydrogen gas were produced.
c) kay didn't correctly mass the precipitate.
d) kay didn't collect all of the precipitate.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, lexybellx3
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3

Chemistry, 21.06.2019 23:30, CaleWort92
Which of the following statements concerning the influence of culture on ethnic identity formation is accurate? a. one will reject ethnic identity if cultural stereotypes are encountered. b. if one’s ethnic city is different from the dominant cultural group, then one’s ethnic identity you will become weekend. c. if an the ethnic group is excepted by dominant culture, then ethnic identity formation can be a difficult process. d. similarity to the dominant culture can determine how easy it is for one to except ethnic differences.
Answers: 2

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 06:00, nikejose11
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3
Do you know the correct answer?
When kay added 30 g of hydrochloric acid (hcl) to 65 g of zn, bubbles appeared and a white precipita...
Questions in other subjects:




Mathematics, 03.07.2020 02:01



Mathematics, 03.07.2020 02:01