Chemistry, 03.10.2019 05:00, genyjoannerubiera
18.1-1. diffusion of methane through helium. a gas of ch4 and he is contained in a tube at 101.32 kpa pressure and 298 k. at one point, the partial pressure of methane is pa1 = 60.79 kpa, and at a point 0.02 m distance away, pa2 = 20.26 kpa. if the total pressure is constant throughout the tube, calculate the flux of ch4 (methane) at steady state for equimolar counterdiffusion.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, happy121906
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
Do you know the correct answer?
18.1-1. diffusion of methane through helium. a gas of ch4 and he is contained in a tube at 101.32 kp...
Questions in other subjects:

Mathematics, 26.05.2021 19:30


Law, 26.05.2021 19:30






Mathematics, 26.05.2021 19:30

Mathematics, 26.05.2021 19:30


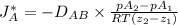
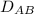 = 0.675 × 10⁻⁴ m²/s (for He-CH4 at 101.32 kPa and 298 K)
= 0.675 × 10⁻⁴ m²/s (for He-CH4 at 101.32 kPa and 298 K)