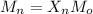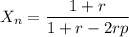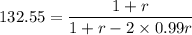Calculate the feed ratio of adipic acid and hexamethylene diamine that should be employed to obtain a polyamide of approximately 15,000 molecular weight at 99.5% conversion. what is the identity of the end groups of this product? do the same calculation for a 19,000-molecular-weight polymer.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, suzymott1562
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1


Chemistry, 22.06.2019 15:20, Tringirl233
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 21:30, MJyoungboy
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
Do you know the correct answer?
Calculate the feed ratio of adipic acid and hexamethylene diamine that should be employed to obtain...
Questions in other subjects:

Chemistry, 04.12.2021 03:10

History, 04.12.2021 03:10


Mathematics, 04.12.2021 03:10

Mathematics, 04.12.2021 03:10


Mathematics, 04.12.2021 03:10


Mathematics, 04.12.2021 03:10

Mathematics, 04.12.2021 03:10