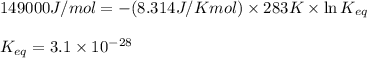Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:30, dorindaramirez0531
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 23.06.2019 10:00, isaiahromero15
The image shows the process of which is used in nuclear power plants. photo attached
Answers: 1

Chemistry, 23.06.2019 11:20, jaidencoolman7072
When using the ideal gas law constant 0.0821, what unit is used for volume? a) galloonb) ouncec) milliliterd) liter
Answers: 1
Do you know the correct answer?
For a certain chemical reaction, the standard gibbs free energy of reaction at 10.0 °c is 149. kj. c...
Questions in other subjects:


English, 31.07.2019 17:30

Mathematics, 31.07.2019 17:30

Computers and Technology, 31.07.2019 17:30




Mathematics, 31.07.2019 17:30


English, 31.07.2019 17:30


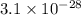
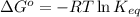
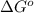 = standard Gibbs free energy = 149. kJ/mol = 149000 J/mol (Conversion factor: 1 kJ = 1000 J )
= standard Gibbs free energy = 149. kJ/mol = 149000 J/mol (Conversion factor: 1 kJ = 1000 J )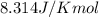
![15^oC=[273+15]K=283K](/tpl/images/0284/3257/82fe2.png)
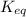 = equilibrium constant at 10°C = ?
= equilibrium constant at 10°C = ?