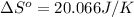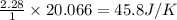Chemistry, 02.10.2019 21:30, dontworry48
Consider the reaction: h2(g) + cl2(g) --> 2hcl(g)
using standard absolute entropies at 298k, calculate the entropy change for the system when 2.28 moles of h2(g)react at standard conditions.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, micvar9646
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 04:30, clairajogriggsk
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 20:00, jalenevoyles
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Do you know the correct answer?
Consider the reaction: h2(g) + cl2(g) --> 2hcl(g)
using standard absolute entropies...
using standard absolute entropies...
Questions in other subjects:

Mathematics, 14.12.2019 22:31

Physics, 14.12.2019 22:31

Mathematics, 14.12.2019 22:31

Mathematics, 14.12.2019 22:31


Biology, 14.12.2019 22:31

Geography, 14.12.2019 22:31

Mathematics, 14.12.2019 22:31

English, 14.12.2019 22:31

Mathematics, 14.12.2019 22:31


 reacts at standard condition is 45.8 J/K
reacts at standard condition is 45.8 J/K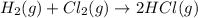
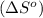 is:
is: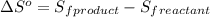
![\Delta S^o=[n_{HCl}\times \Delta S_f^0_{(HCl)}]-[n_{H_2}\times \Delta S_f^0_{(H_2)}+n_{Cl_2}\times \Delta S_f^0_{(Cl_2)}]](/tpl/images/0284/2286/d7d45.png)
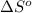 = entropy change of reaction = ?
= entropy change of reaction = ?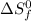 = standard entropy of formation
= standard entropy of formation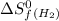 = 130.684 J/mol.K
= 130.684 J/mol.K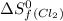 = 223.066 J/mol.K
= 223.066 J/mol.K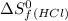 = 186.908 J/mol.K
= 186.908 J/mol.K![\Delta S^o=[2mole\times (186.908J/K.mole)]-[1mole\times (130.684J/K.mole)+1mole\times (223.066J/K.mole)}]](/tpl/images/0284/2286/55b6e.png)