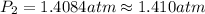Answers: 1
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 09:30, janetexcoelho
What does the mass of 0.7891 mol of ferric oxide (fe2o3)
Answers: 1
Do you know the correct answer?
The vapor pressure of water is 1.00 atm at 373 k, and the enthalpy of vaporization is 40.68 kj mol!...
Questions in other subjects:


Mathematics, 07.04.2020 22:41


Mathematics, 07.04.2020 22:41

English, 07.04.2020 22:41


Business, 07.04.2020 22:41

History, 07.04.2020 22:41


Mathematics, 07.04.2020 22:41


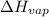 of the reaction, we use clausius claypron equation, which is:
of the reaction, we use clausius claypron equation, which is:![\ln(\frac{P_2}{P_1})=\frac{\Delta H_{vap}}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0284/2756/b9a49.png)
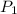 = vapor pressure at temperature
= vapor pressure at temperature 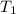
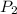 = vapor pressure at temperature
= vapor pressure at temperature 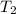
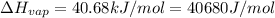
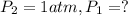
![\ln(\frac{1 atm}{P_1})=\frac{40680 J/mol}{8.314J/mol.K}[\frac{1}{363}-\frac{1}{373}]](/tpl/images/0284/2756/cf798.png)
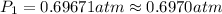
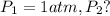
![\ln(\frac{P_2}{1 atm})=\frac{40680 J/mol}{8.314J/mol.K}[\frac{1}{373}-\frac{1}{383}]](/tpl/images/0284/2756/01f21.png)