
Chemistry, 02.10.2019 22:00, HELPPPPPfawfds
You need to prepare 0.2 liters of a 0.1 m solution of a phosphate buffer with a ph of 7.2. if you have solid k2hpo4 (fw=136.09) and 6m hcl or 6 m naoh describe how you would prepare this buffer. note: pka values for phosphoric acid are 2.15, 6.86, and 12.35

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
Do you know the correct answer?
You need to prepare 0.2 liters of a 0.1 m solution of a phosphate buffer with a ph of 7.2. if you ha...
Questions in other subjects:


Biology, 09.01.2020 04:31

History, 09.01.2020 04:31

Chemistry, 09.01.2020 04:31


History, 09.01.2020 04:31

Mathematics, 09.01.2020 04:31

Mathematics, 09.01.2020 04:31



![\frac{[HPO_{4}^{2-}]}{[H_{2}PO_{4}^-]}](/tpl/images/0284/2917/12fd1.png)
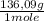 = 2,722 g
= 2,722 g



