Chemistry, 02.10.2019 22:00, TropicalFan
Certain atoms emit photons of light with an energy of 3.820 ✕ 10−19 j. calculate the frequency (in hz) and wavelength (in nm) of one of these photons.
frequency hz
and
wavelength nm
what is the total energy (in kj) in 1 mole of these photons?
kj

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, arodavoarodavo
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 13:00, devontemiles8868
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1
Do you know the correct answer?
Certain atoms emit photons of light with an energy of 3.820 ✕ 10−19 j. calculate the frequency (in h...
Questions in other subjects:


Chemistry, 06.04.2021 19:50


English, 06.04.2021 19:50





History, 06.04.2021 19:50

Mathematics, 06.04.2021 19:50


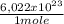 = 230040 J/mole ≡ 230,0 kJ/mole
= 230040 J/mole ≡ 230,0 kJ/mole