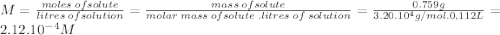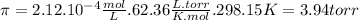Chemistry, 02.10.2019 22:00, veronica25681
Astarch has a molar mass of 3.20 x 104 g/mol. if 0.759 g of this starch is dissolved in 112 ml of solution, what is the osmotic pressure, in torr, at 25.00 oc?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, Apple557
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1


Chemistry, 22.06.2019 23:30, shukriabdisabrie
Match each statement with the state of matter it describes
Answers: 3
Do you know the correct answer?
Astarch has a molar mass of 3.20 x 104 g/mol. if 0.759 g of this starch is dissolved in 112 ml of so...
Questions in other subjects:

Spanish, 10.09.2021 20:40



Mathematics, 10.09.2021 20:40


Mathematics, 10.09.2021 20:40

History, 10.09.2021 20:40



Mathematics, 10.09.2021 20:40