
Chemistry, 02.10.2019 22:00, hunterwilliams375
What is the vapor pressure at 23 oc of a solution of 1.20 g of naphthalene, c10h8, in 25.6 g of benzene, c6h6? . the vapor pressure of pure benzene at 23 oc is 86.0 mm hg; the vapor pressure of naphthalene can be neglected. calculate the vapor-pressure lowering of the solution.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1


Chemistry, 22.06.2019 17:10, gungamer720
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1
Do you know the correct answer?
What is the vapor pressure at 23 oc of a solution of 1.20 g of naphthalene, c10h8, in 25.6 g of benz...
Questions in other subjects:


Chemistry, 25.11.2019 23:31

Mathematics, 25.11.2019 23:31

Mathematics, 25.11.2019 23:31

Business, 25.11.2019 23:31



Biology, 25.11.2019 23:31

Health, 25.11.2019 23:31

Mathematics, 25.11.2019 23:31


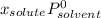 (1)
(1)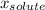 is molar fraction of solute
is molar fraction of solute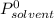 is the capor pressure of the pure solvent (86,0 mmHg)
is the capor pressure of the pure solvent (86,0 mmHg)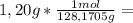 9,36x10⁻³ moles solute
9,36x10⁻³ moles solute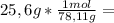 0,328 moles solvent
0,328 moles solvent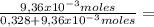 0,0278
0,0278



