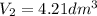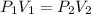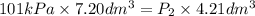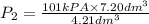Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, smhrosepetals
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2



Chemistry, 23.06.2019 05:00, jjoyner
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
Do you know the correct answer?
Synthesis gas consists of a mixture of h2 and co. calculate at 101kpa is compressed at constant temp...
Questions in other subjects:


Computers and Technology, 23.02.2021 17:50


Mathematics, 23.02.2021 17:50

Social Studies, 23.02.2021 17:50

Mathematics, 23.02.2021 17:50

Chemistry, 23.02.2021 17:50


Social Studies, 23.02.2021 17:50


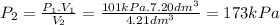
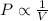 ; at constant T
; at constant T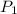 = 101 kPa,
= 101 kPa, 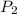 = ?
= ?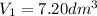 ,
,