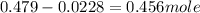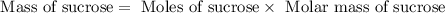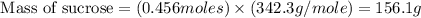The hydrolysis of sucrose (c12h22011) into glucose and fructose in acidic solution is a first-order reaction with a rate constant of 1.8 x 10-45-1 at 25°c. determine the mass (g) of sucrose that is consumed when 2.15 l of a 0.223 m sucrose solution is allowed to react for 282 minutes. enter your answer as an integer. previous next not saved submit qu.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Do you know the correct answer?
The hydrolysis of sucrose (c12h22011) into glucose and fructose in acidic solution is a first-order...
Questions in other subjects:

Social Studies, 17.10.2020 09:01

History, 17.10.2020 09:01

Chemistry, 17.10.2020 09:01

Biology, 17.10.2020 09:01


History, 17.10.2020 09:01

Mathematics, 17.10.2020 09:01



English, 17.10.2020 09:01


![[C_t]=[C_o]e^{-kt}](/tpl/images/0284/1096/86a58.png)
![[C_t]](/tpl/images/0284/1096/81c01.png) = concentration of sucrose at time 't'
= concentration of sucrose at time 't'![[C_o]](/tpl/images/0284/1096/61f0a.png) = concentration of sucrose at time '0' = 0.223 M
= concentration of sucrose at time '0' = 0.223 M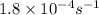
![[C_t]=(0.223)\times e^{-(1.8\times 10^{-4})\times (16920)}](/tpl/images/0284/1096/fccf3.png)
![[C_t]=0.0106M](/tpl/images/0284/1096/bb844.png)
![n_o=[C_o]\times V=0.223M\times 2.15L=0.479moles](/tpl/images/0284/1096/1c118.png)
![n_t=[C_t]\times V=0.0106M\times 2.15L=0.0228moles](/tpl/images/0284/1096/96015.png)