
Chemistry, 02.10.2019 21:00, gorbyalexis
Calculate the equilibrium concentrations of n2o4 and no2 at 25 ∘c in a vessel that contains an initial n2o4 concentration of 0.0654 m . the equilibrium constant kc for the reaction n2o4(g)⇌2no2(g) is 4.64×10−3 at 25 ∘c.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1


Chemistry, 22.06.2019 13:30, justinerodriguz2878
What are the major types of a chemical compound
Answers: 2

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
Do you know the correct answer?
Calculate the equilibrium concentrations of n2o4 and no2 at 25 ∘c in a vessel that contains an initi...
Questions in other subjects:





Mathematics, 15.04.2020 04:04






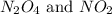 are 0.0164 M and 0.0572 M
are 0.0164 M and 0.0572 M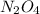 = 0.0654 M
= 0.0654 M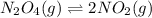
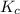 for above reaction follows:
for above reaction follows:![K_c=\frac{[NO_2]_{eq}^2}{[N_2O_4]_{eq}}](/tpl/images/0284/1377/77dc1.png)
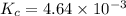
![[NO_2]_{eq}=2x](/tpl/images/0284/1377/0bbcc.png)
![[N_2O_4]_{eq}=0.0654-x](/tpl/images/0284/1377/db879.png)
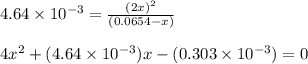
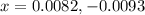
![[NO_2]_{eq}=2x=2(0.0082)=0.0164M](/tpl/images/0284/1377/53c5f.png)
![[N_2O_4]_{eq}=0.0654-x=(0.0654-0.0082)=0.0572M](/tpl/images/0284/1377/125d4.png)




