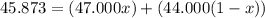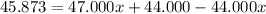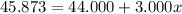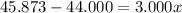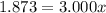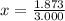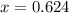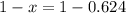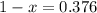Chemistry, 02.10.2019 20:10, BigGirlsTheBest
An element has only two naturally occurring isotopes. you know that isotope 1 has a mass of 44.000 amu and isotope 2 has a mass of 47.000 amu. if the average atomic mass is 45.873 amu, what are the natural abundances in percent for the two isotopes? 14.00m

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Bradgarner772
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1


Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 16:00, annsmith66
What statement goes against the kinetic theory of gases
Answers: 1
Do you know the correct answer?
An element has only two naturally occurring isotopes. you know that isotope 1 has a mass of 44.000 a...
Questions in other subjects:

Mathematics, 25.03.2020 18:56

Computers and Technology, 25.03.2020 18:56

Social Studies, 25.03.2020 18:56



Mathematics, 25.03.2020 18:57

Mathematics, 25.03.2020 18:57

Biology, 25.03.2020 18:57