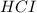Hydrocyanic acid, hcn, is a weak acid. (a) write the chemical equation for the dissociation of hcn in water. (b) identify the brønsted-lowry conjugate acid-base pairs in the equation above. (c) write the chemical equation for the reaction of hcn with naoh. (d) write the chemical equation for dissociation of nacn in water. (e) write the chemical equation for the reaction of nacn and hci.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, zaehairston78531
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1


Chemistry, 22.06.2019 04:30, mamabates181981
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1
Do you know the correct answer?
Hydrocyanic acid, hcn, is a weak acid. (a) write the chemical equation for the dissociation of hcn i...
Questions in other subjects:

History, 22.11.2019 20:31

Mathematics, 22.11.2019 20:31


History, 22.11.2019 20:31






Physics, 22.11.2019 20:31


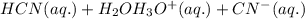
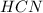 : acid
: acid 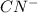 :conjugate base.
:conjugate base.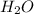 : base
: base 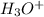 : conjugate acid.
: conjugate acid.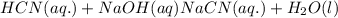
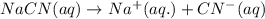
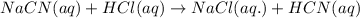
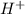 ions in their aqueous states.
ions in their aqueous states.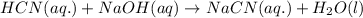
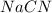 in water.
in water.