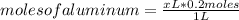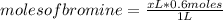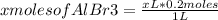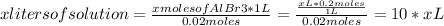Chemistry, 02.10.2019 19:30, erinwebsterrr
Abeaker holds xliters of 0.2m albr3. give answers to parts a through din terms of x. a) how many moles of aluminum ions are there in this solution? b) how many moles of bromide ions are there in this solution? c) how much water must you add if want to dilute the original solution to a concentration of 0.02 m?

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2
Do you know the correct answer?
Abeaker holds xliters of 0.2m albr3. give answers to parts a through din terms of x. a) how many mol...
Questions in other subjects:


Geography, 16.08.2019 18:10

History, 16.08.2019 18:10



Geography, 16.08.2019 18:10

Biology, 16.08.2019 18:10


Mathematics, 16.08.2019 18:10

Mathematics, 16.08.2019 18:10