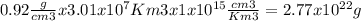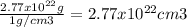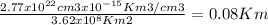The total volume of ice in the antarctic is about 3.01 x 107 km3. if all the ice in the antarctic were to melt completely, estimate the rise, h, in sea level that would result from the additional liquid water entering the oceans. the densities of ice and fresh water are 0.92 g/cm3 and 1.0 g/cm3, respectively. assume that the oceans of the world cover an area, a, of about 3.62 x 108 km2 and that the increase in volume of the oceans can be calculated as a x h.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, alaynagrace1111
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 08:40, sugakookies1
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2
Do you know the correct answer?
The total volume of ice in the antarctic is about 3.01 x 107 km3. if all the ice in the antarctic we...
Questions in other subjects:


Mathematics, 01.02.2020 13:44


English, 01.02.2020 13:44




Mathematics, 01.02.2020 13:44

Mathematics, 01.02.2020 13:44

Mathematics, 01.02.2020 13:44