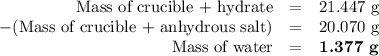Analo387: percent water in a hydrate
3. the mass of a crucible and a hydrated salt was found...

Chemistry, 02.10.2019 17:30, abdullaketbi71
Analo387: percent water in a hydrate
3. the mass of a crucible and a hydrated salt was found to be 21.447 g. the mass of the crucible and the
anhydrous salt was 20.070 g. the mass of the crucible was 17.985 g.
(1) calculate the mass of the hydrate heated.
(2) calculate the mass of water lost from the hydrate during heating.
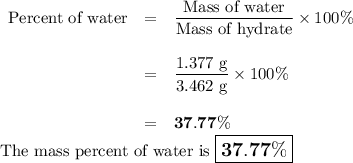
(3) calculate the percent water in the hydrate.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, porkhappycom
This line graph compares the growth of plants that were kept in the sun for different amounts of time.a) on day 7, the plants kept in the sun for 3 hours were how tall? b) on day 7, the plants kept in the sun for 6 hours were how tall? c) on day 10, the plants kept in the sun for 9 hours were how tall? d) on day 11, the plant that was grown with 1 hour of sunlight was how tall? e) based on the graph, the plant grows best in what amount of sunlight?
Answers: 1



Chemistry, 22.06.2019 12:20, missayers172
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3
Do you know the correct answer?
Questions in other subjects:






Chemistry, 06.05.2020 06:09



Mathematics, 06.05.2020 06:10