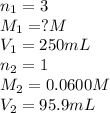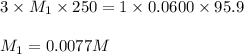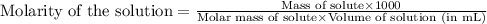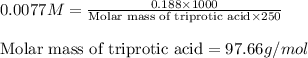Chemistry, 02.10.2019 02:00, Trevon0906
An analytical chemist weighs out 0.188 g of an unknown triprotic acid into a 250 ml volumetric flask and dilutes to the mark with distilled water. he then titrates this solution with 0.0600 m naoh solutions. when the titration reaches the equivalence point, the chemist finds he has added 95.9 ml of naoh solution.
calculate the molar mass of the unknown acid.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1

Chemistry, 22.06.2019 14:40, elawnnalewis4855
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
Do you know the correct answer?
An analytical chemist weighs out 0.188 g of an unknown triprotic acid into a 250 ml volumetric flask...
Questions in other subjects:


English, 08.06.2020 07:57


History, 08.06.2020 07:57







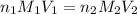
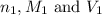 are the n-factor, molarity and volume of triprotic acid
are the n-factor, molarity and volume of triprotic acid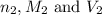 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.