
Chemistry, 02.10.2019 00:30, XxKaitlynnxX
At equilibrium, the concentrations in this system were found to be [n2]=[o2]=0.100 m and [no]=0.400 m. n2(g)+o2(g)↽−−⇀2no(g) if more no is added, bringing its concentration to 0.700 m, what will the final concentration of no be after equilibrium is re‑established?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Do you know the correct answer?
At equilibrium, the concentrations in this system were found to be [n2]=[o2]=0.100 m and [no]=0.400...
Questions in other subjects:

Mathematics, 26.02.2021 22:20









Chemistry, 26.02.2021 22:20


![K = \frac{[C]^{c}.[D]^{d}}{[A]^{a}.[B]^{b}}](/tpl/images/0281/3856/d6241.png)
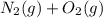 ⇄ 2 NO (g), the equilibrium constant could be calculated as well:
⇄ 2 NO (g), the equilibrium constant could be calculated as well:![K = \frac{[NO]^{2}}{[O_{2}].[N_{2}]}](/tpl/images/0281/3856/92022.png)
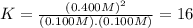
![[N_{2}] = 0.100 M; [O_{2}] = 0.100 M; [NO] = 0.700 M](/tpl/images/0281/3856/59e72.png)
![[N_{2}] = 0.100 M + x; [O_{2}] = 0.100 M + x; [NO] = 0.700 M - 2x](/tpl/images/0281/3856/782eb.png)
![K = \frac{[NO]^{2}}{[O_{2}].[N_{2}]} = 16](/tpl/images/0281/3856/cb079.png)
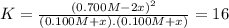
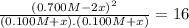
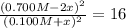
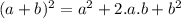
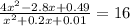
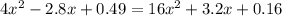
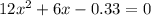
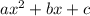 is:
is:![x_{1} =\frac{-b+\sqrt[]{4.a.c} }{2.a} \\x_{2} =\frac{-b-\sqrt[]{4.a.c} }{2.a}](/tpl/images/0281/3856/fdc5b.png)
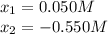
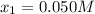
![[N_{2}] = 0.100 M + x = 0.150 M; [O_{2}] = 0.100 M + x = 0.150 M; [NO] = 0.700 M - 2x = 0.600 M](/tpl/images/0281/3856/a4921.png)
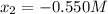
![[N_{2}] = 0.100 M + x = -0.450 M; [O_{2}] = 0.100 M + x = -0.450 M; [NO] = 0.700 M - 2x = 1.800 M](/tpl/images/0281/3856/d9901.png)
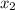 isn´t a possible answer because it results in negative equilibrium concentrations of the reagents. Then Case
isn´t a possible answer because it results in negative equilibrium concentrations of the reagents. Then Case 



