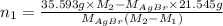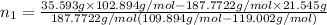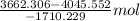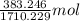Chemistry, 01.10.2019 22:00, GodlyGamer8239
Both kbr and nabr are soluble ionic compounds and fully dissociate in aqueous solution. a 21.545-g mixture of kbr and nabr is dissolved in water, then a solution of agno3 is added so that all of the bromine present is converted to solid agbr. the agbr product is dried and found to have a mass of 35.593 g. what mass of kbr was present in the original mixture?

Answers: 1
Similar questions



Physics, 28.10.2019 21:31, AnkitDavid1616
Answers: 2
Do you know the correct answer?
Both kbr and nabr are soluble ionic compounds and fully dissociate in aqueous solution. a 21.545-g m...
Questions in other subjects:

Mathematics, 10.12.2020 06:10

English, 10.12.2020 06:10



Mathematics, 10.12.2020 06:10


History, 10.12.2020 06:10


Mathematics, 10.12.2020 06:10

History, 10.12.2020 06:10


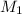 and NaBr is
and NaBr is 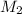 .
.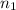 and
and 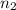 .
.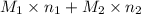 = 21.545 g
= 21.545 g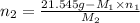
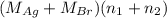 = 35.593 g
= 35.593 g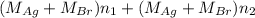 = 35.593 g
= 35.593 g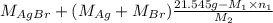 = 35.593 g
= 35.593 g