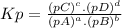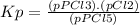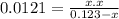Chemistry, 01.10.2019 19:00, sierravick123owr441
The equilibrium constant (k p) for the interconversion of pcl 5 and pcl 3 is 0.0121: pcl 5 (g) pcl 3 (g) cl 2 (g) a vessel is charged with pcl 5, giving an initial pressure of 0.123 atm. at equilibrium, the partial pressure of pcl 3 is atm.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 10:00, Cythina2007
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1


Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
Do you know the correct answer?
The equilibrium constant (k p) for the interconversion of pcl 5 and pcl 3 is 0.0121: pcl 5 (g) pcl...
Questions in other subjects:




Mathematics, 10.12.2020 01:50

Medicine, 10.12.2020 01:50


Mathematics, 10.12.2020 01:50


Biology, 10.12.2020 01:50

German, 10.12.2020 01:50


![Kc = \frac{[C]^c.[D]^d}{[A]^a.[B]^b}](/tpl/images/0280/5110/4ea0c.png)