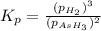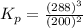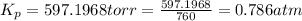Chemistry, 01.10.2019 04:20, gadgetady5699
The gas arsine (ash3) decomposes as follows: 2ash3(g) < > 2as(s) + 3h2(g) in an experiment pure ash3(g) was placed in an empty, rigid, sealed flask at a pressure of 392.0 torr. after 48 h the pressure in the flask was observed to be constant at 488.0 torr. a. calculate the equilibrium pressure of h2(g). b. calculate kp for this reaction.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2


Chemistry, 22.06.2019 19:00, Jasoncookies23
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
Do you know the correct answer?
The gas arsine (ash3) decomposes as follows: 2ash3(g) < > 2as(s) + 3h2(g) in an experiment p...
Questions in other subjects:



Biology, 26.07.2019 00:30





Computers and Technology, 26.07.2019 00:30



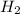 gas is, 288 torr
gas is, 288 torr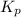 for this reaction is, 0.786 atm
for this reaction is, 0.786 atm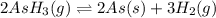
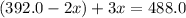
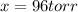
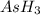 at equilibrium = (392.0-2x) = [392.0-2(96)] = 200 torr
at equilibrium = (392.0-2x) = [392.0-2(96)] = 200 torr