Background info:
the standard enthalpy of formation (δh∘f) is the enthalpy change that occurs...

Chemistry, 19.08.2019 10:30, terryonsavage543
Background info:
the standard enthalpy of formation (δh∘f) is the enthalpy change that occurs when exactly1 mol of a compound is formed from its constituent elements under standard conditions. the standard conditions are 1 atm pressure, a temperature of 25 ∘c , and all the species present at a concentration of 1 m . a "standard enthalpies of formation table" containing δh∘f values might look something like this: substanceδh∘fh(g)218 kj/molh2(g)0 kj/molba(s)0 kj/molba2+(aq)−538.4 kj/molc(g)71 kj/molc(s)0 kj/moln(g)473 kj/molo2(g)0 kj/molo(g)249 kj/mols2(g)129 kj/mol
question:
what is the balanced chemical equation for the reaction used to calculate δh∘f of baco3(s)? if fractional coefficients are required, enter them as a fraction (i. e. 1/3). indicate the physical states using the abbreviation (s), (l), or (g) for solid, liquid, or gas, respectively. use (aq) for aqueous solution.
express answer as a chemical equation. explain for me !

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 08:30, vett072804
Benzonitrile (c6h5cn) is reduced to two different products depending on the reducing agent used. treatment with lithium aluminum hydride followed by water forms k, which has a molecular ion in its mass spectrum at 107 and the following ir absorptions: 3373, 3290, 3062, 2920, and 1600 cm-1. treatment with a milder reducing agent forms l, which has a molecular ion in its mass spectrum at 106 and the following ir absorptions: 3086, 2850, 2820, 2736, 1703, and 1600 cm-1. l shows fragments in its mass spectrum at m/z = 105 and 77. propose structures for k and l and choose an explanation for how this could be concluded.
Answers: 3
Do you know the correct answer?
Questions in other subjects:










Computers and Technology, 04.09.2019 18:30


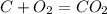
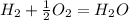
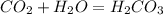
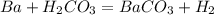
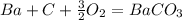 , using hydrogen as a catalyst.
, using hydrogen as a catalyst.



