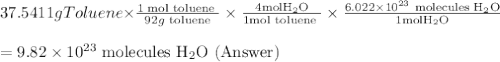One of the compounds used to increase the octane rating of gasoline is toluene (pictured). suppose 43.3 ml of toluene (d = 0.867 g/ml) is consumed when a sample of gasoline burns in air. how many grams of oxygen are needed for complete combustion of the toluene? (a) how many grams of oxygen are needed for complete combustion of the toluene? g (b) how many total moles of gaseous products form? mol (c) how many molecules of water vapor form?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, dustinquiz255
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 19:00, cindyroxana229
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 20:00, Chynadoll94
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 21:00, melissalopez12
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1
Do you know the correct answer?
One of the compounds used to increase the octane rating of gasoline is toluene (pictured). suppose 4...
Questions in other subjects:

English, 01.12.2020 23:50

English, 01.12.2020 23:50


Mathematics, 01.12.2020 23:50


English, 01.12.2020 23:50


English, 01.12.2020 23:50



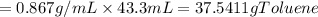
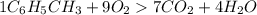
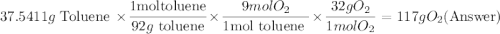
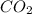 gas and 4 moles of
gas and 4 moles of 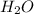 Vapour
Vapour
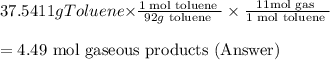
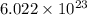 molecules
molecules