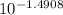Chemistry, 30.09.2019 21:00, geunagillis1
Abuffered solution containing dissolved aniline, c6h5nh2, and aniline hydrochloride, c6h5nh3cl, has a ph of 5.75.
a) determine the concentration of c6h5nh3+ in the solution if the concentration of c6h5nh2 is 0.245 m. the pkb of aniline is 9.13.
[c6h5nh3+]= m
b) calculate the change in ph of the solution, \delta ph, if 0.360g naoh is added to the buffer for a final volume of 1.50l.
\deltaph=

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, fespinoza019
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 05:30, jzjajsbdb8035
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 09:20, lanaiheart7
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2
Do you know the correct answer?
Abuffered solution containing dissolved aniline, c6h5nh2, and aniline hydrochloride, c6h5nh3cl, has...
Questions in other subjects:


Biology, 03.03.2021 05:20

Chemistry, 03.03.2021 05:20



Mathematics, 03.03.2021 05:20

Mathematics, 03.03.2021 05:20


Mathematics, 03.03.2021 05:20

English, 03.03.2021 05:20


![pH = pKa + log\frac{[A^-]}{[HA]}](/tpl/images/0277/5492/19c19.png)