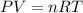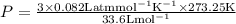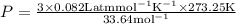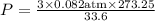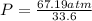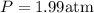Chemistry, 30.09.2019 04:30, superstarsara5ouh83x
3.0 mol of gas occupy 33.6 l at a temperature of 0°c. what is the pressure of the gas?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, nique0808
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 23.06.2019 07:30, jonquil201
Using this reversible reaction, answer the questions below: n2o4 2no2 (colorless) (reddish-brown) -as the temperature increased, what happened to the n2o4 concentration? -was the formation of reactants or products favored by the addition of heat? -which reaction is exothermic? right to left or left to right? -if the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct? n2o4 2no2 + 14 kcal n2o4 2no2, hr = +14 kcal n2o4 + 14 kcal 2no2 n2o4 2no2, hr = -14 kcal
Answers: 1

Chemistry, 23.06.2019 10:30, cjtambasco
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 3.75 mol fe and 8.70 mol nio(oh) react?
Answers: 1

Chemistry, 23.06.2019 13:40, gtamods402
Which of the following volumes is the smallest? a) one microliter b)one deciliter d)one liter c)one milliliter
Answers: 2
Do you know the correct answer?
3.0 mol of gas occupy 33.6 l at a temperature of 0°c. what is the pressure of the gas?...
Questions in other subjects:






English, 31.01.2020 10:05

Mathematics, 31.01.2020 10:05


English, 31.01.2020 10:05

English, 31.01.2020 10:05