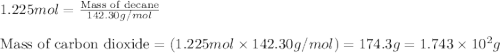Chemistry, 28.09.2019 03:30, kyramks421
Various members of a class of compounds, alkenes, react with hydrogen to produce a corresponding alkane. termed hydrogenation, this type of reaction is used to produce products such as margarine. a typical hydrogenation reaction is c10h20() + h2(g) → c10h22(5) decene decane how much decane can be produced in a reaction of excess decene with 2.45 g hydrogen? give your answer in scientific notation. o *10 g decane

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 04:20, lindseysmith9522
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 09:30, jewelz5887
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
Do you know the correct answer?
Various members of a class of compounds, alkenes, react with hydrogen to produce a corresponding alk...
Questions in other subjects:

English, 18.03.2021 20:10


English, 18.03.2021 20:10



Social Studies, 18.03.2021 20:10


Mathematics, 18.03.2021 20:10



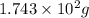
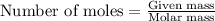 ......(1)
......(1)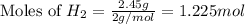
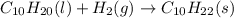
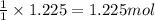 of decane
of decane