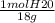Chemistry, 28.09.2019 03:10, marendt2014
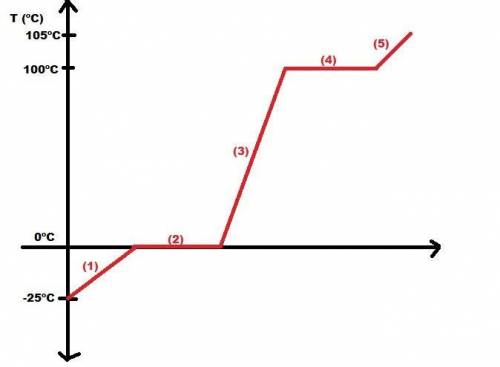
Calculate the amount of heat in kj that is required to heat 25.0 g of ice from -25 °c to 105 °c in a closed vessel and sketch a heating curve for the process. the specific heat of ice is 2.11 j/(g. "c); 4.18 j/g. "c) for water, 2.00 j/g. "c. ahus for water is 6,01 kj/mol; ahp for water = 40.67 kj/mol.

Answers: 2
Other questions on the subject: Chemistry

Do you know the correct answer?
Calculate the amount of heat in kj that is required to heat 25.0 g of ice from -25 °c to 105 °c in a...
Questions in other subjects:


Mathematics, 12.02.2020 06:22

Engineering, 12.02.2020 06:22

Mathematics, 12.02.2020 06:22


Mathematics, 12.02.2020 06:22



Mathematics, 12.02.2020 06:22


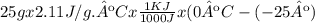
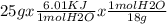
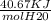 x
x