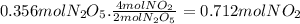Chemical equations
instructions: solve the following chemical equations.
fo...

Chemistry, 28.09.2019 02:30, MahiraBashir
Chemical equations
instructions: solve the following chemical equations.
for the following reaction, calculate how many moles of no2forms when 0.356 moles of the reactant completely reacts. 2 n2o5(g) > 4 no2(g) + 02(g)

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Do you know the correct answer?
Questions in other subjects:


Mathematics, 30.11.2021 08:50




Chemistry, 30.11.2021 08:50


Mathematics, 30.11.2021 08:50


Mathematics, 30.11.2021 08:50