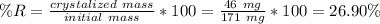Chemistry, 28.09.2019 02:20, kingofguns2826
the solubility of acetanilide is 12.8 g in 100 ml of ethanol at 0 ∘c, and 46.4 g in 100 ml of ethanol at 60 ∘c. what is the maximum percent recovery that can be achieved for the recrystallization of acetanilide from ethanol?
a student was given a sample of crude acetanilide to recrystallize. the initial mass of the the crude acetanilide was 171 mg. the mass after recrystallization was 125 mg.
calculate the percent recovery from recrystallization.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, artemiscrock77041
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1


Chemistry, 22.06.2019 12:20, missayers172
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
Do you know the correct answer?
the solubility of acetanilide is 12.8 g in 100 ml of ethanol at 0 ∘c, and 46.4 g in 100 ml of ethano...
Questions in other subjects:


Mathematics, 01.01.2020 02:31



Mathematics, 01.01.2020 02:31



Mathematics, 01.01.2020 02:31