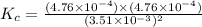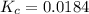Gaseous hydrogen iodide is placed in a closed container at 425°c, where it partially decomposes to hydrogen and iodine: 2hi(g)⇌h₂(g)+i₂(g) at equilibrium it is found that [hi]= 3.51×10⁻³ m, [h₂]= 4.76×10⁻⁴ m, and [i₂]= 4.76×10⁻⁴ m.
what is the value of  at this temperature? express the equilibrium constant to three significant digits.
at this temperature? express the equilibrium constant to three significant digits.

Answers: 3
Similar questions

Chemistry, 30.07.2019 01:20, brandyjune9546
Answers: 3

Chemistry, 01.08.2019 01:10, hunterkerlin20p3l6ff
Answers: 1

Chemistry, 08.08.2019 06:10, kaylee0424
Answers: 1

Chemistry, 20.08.2019 23:30, eyeneedalife
Answers: 1
Do you know the correct answer?
Gaseous hydrogen iodide is placed in a closed container at 425°c, where it partially decomposes to h...
Questions in other subjects:

Mathematics, 06.07.2019 15:40


Arts, 06.07.2019 15:40



Mathematics, 06.07.2019 15:40



French, 06.07.2019 15:40

Arts, 06.07.2019 15:40


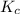 at this temperature is 0.0184
at this temperature is 0.0184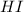 at equilibrium =
at equilibrium = 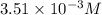
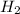 at equilibrium =
at equilibrium = 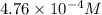
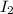 at equilibrium =
at equilibrium = 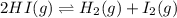
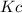 will be,
will be,![K_c=\frac{[H_2][I_2]}{[HI]^2}](/tpl/images/0269/8146/ef85e.png)