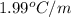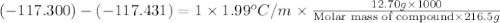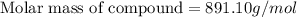Chemistry, 28.09.2019 01:30, emrecaga1992
The freezing point of ethanol, ch3ch2oh, is -117.300 °c at 1 atmosphere. kf(ethanol) = 1.99 °c/m
in a laboratory experiment, students synthesized a new compound and found that when 12.70 grams of the compound were dissolved in 216.5 grams of ethanol, the solution began to freeze at -117.431 °c. the compound was also found to be nonvolatile and a non-electrolyte.
what is the molecular weight they determined for this compound?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chameleonsarelife
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3


Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
Do you know the correct answer?
The freezing point of ethanol, ch3ch2oh, is -117.300 °c at 1 atmosphere. kf(ethanol) = 1.99 °c/m
Questions in other subjects:


Mathematics, 21.10.2020 05:01

Health, 21.10.2020 05:01


Mathematics, 21.10.2020 05:01

Biology, 21.10.2020 05:01




English, 21.10.2020 05:01



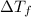 = change in freezing point
= change in freezing point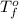 = temperature of pure ethanol =
= temperature of pure ethanol = 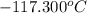
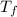 = temperature of solution =
= temperature of solution = 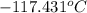
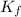 = freezing point constant of ethanol =
= freezing point constant of ethanol =