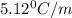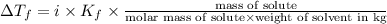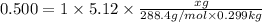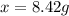How many grams of testosterone, c19h28o2 (288.4 g/mol), must be dissolved in 299.0 grams of benzene to reduce the freezing point by 0.500°c ? refer to the table for the necessary boiling or freezing point constant.
solvent
formula
kb (°c/m)
kf (°c/m)
water
h2o
0.512
1.86
ethanol
ch3ch2oh
1.22
1.99
chloroform
chcl3
3.67
benzene
c6h6
2.53
5.12
diethyl ether
ch3ch2och2ch3
2.02
? g testosterone.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 23.06.2019 01:00, daniel1480
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
Do you know the correct answer?
How many grams of testosterone, c19h28o2 (288.4 g/mol), must be dissolved in 299.0 grams of benzene...
Questions in other subjects:

Physics, 22.12.2020 17:50

Mathematics, 22.12.2020 17:50


English, 22.12.2020 17:50

History, 22.12.2020 17:50

English, 22.12.2020 17:50


Mathematics, 22.12.2020 17:50

English, 22.12.2020 17:50


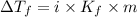
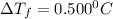 = Depression in freezing point
= Depression in freezing point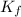 = freezing point constant of benzene=
= freezing point constant of benzene=