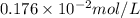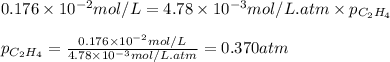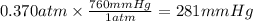Chemistry, 28.09.2019 01:30, MIAkwicc39
What partial pressure of c2h4 gas (in mm hg) is required to maintain a solubility of 4.92×10-2 g/l in water at 25 °c? kh for c2h4 at 25 °c is 4.78×10-3 mol/l·atm.

Answers: 3
Similar questions

Chemistry, 09.07.2019 08:30, yessy73
Answers: 1


Chemistry, 30.07.2019 17:30, 0055babs
Answers: 1

Chemistry, 04.09.2019 19:30, u8p4
Answers: 1
Do you know the correct answer?
What partial pressure of c2h4 gas (in mm hg) is required to maintain a solubility of 4.92×10-2 g/l i...
Questions in other subjects:

Geography, 10.10.2019 03:00

History, 10.10.2019 03:00

Mathematics, 10.10.2019 03:00


Mathematics, 10.10.2019 03:00


Chemistry, 10.10.2019 03:00




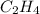 is 281 mmHg
is 281 mmHg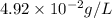
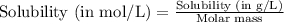
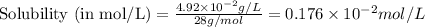
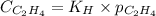
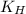 = Henry's constant =
= Henry's constant = 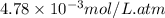
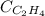 = molar solubility of ethene gas =
= molar solubility of ethene gas =