
Achemistry student needs 50.0 g of methyl acetate for an experiment. by consulting the crc handbook of chemistry and physics, the student discovers that the density of methyl acetate is 0.934 g. cm . calculate the volume of methyl acetate the student should pour out. round your answer to 3 significant digits. x s ?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:50, kukisbae
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 02:20, kristieroth1
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Do you know the correct answer?
Achemistry student needs 50.0 g of methyl acetate for an experiment. by consulting the crc handbook...
Questions in other subjects:



Mathematics, 10.12.2020 18:40



English, 10.12.2020 18:40


English, 10.12.2020 18:40



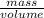
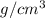 .
.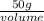
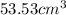
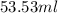 (as 1
(as 1 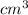 = 1 mL)
= 1 mL)



