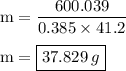Chemistry, 27.09.2019 17:30, Annaborden02
Ablock of copper of unknown mass has an initial temperature of 65.4 ∘c. the copper is immersed in a beaker containing 95.7 g of water at 22.7 ∘c. when the two substances reach thermal equilibrium, the final temperature is 24.2 ∘c. what is the mass of the copper block? express your answer in grams to three significant figures.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, xoxokaydavis5837
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2


Chemistry, 22.06.2019 22:30, COOLIOMARIS
What three things does a balanced equation show you?
Answers: 1
Do you know the correct answer?
Ablock of copper of unknown mass has an initial temperature of 65.4 ∘c. the copper is immersed in a...
Questions in other subjects:


History, 06.07.2019 01:00


History, 06.07.2019 01:00

Social Studies, 06.07.2019 01:00

Chemistry, 06.07.2019 01:00

Health, 06.07.2019 01:00


Mathematics, 06.07.2019 01:00

Mathematics, 06.07.2019 01:00