
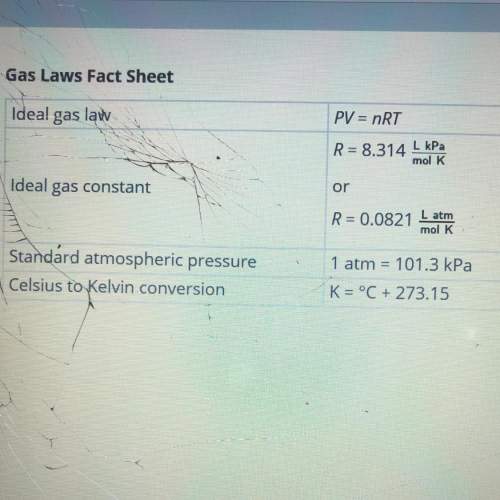
Calculate the number of moles of nitrogen required to fill the airbag. show your work. assume that the nitrogen produced by the chemical reaction is at temperature of 495c and that nitrogen gas behaves like an ideal gas. use this fact sheet to review the ideal gas law.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, KKHeffner02
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1
Do you know the correct answer?
Calculate the number of moles of nitrogen required to fill the airbag. show your work. assume that t...
Questions in other subjects:

Social Studies, 08.11.2019 19:31



Mathematics, 08.11.2019 19:31

Biology, 08.11.2019 19:31

Health, 08.11.2019 19:31



Mathematics, 08.11.2019 19:31






