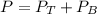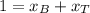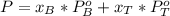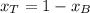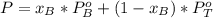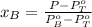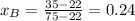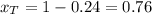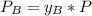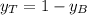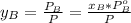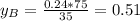Chemistry, 27.09.2019 04:10, chamarabrown9260
A) whatis the composition in mole fractions of a solution of benzene and toluene that has a vapor pressure of 35 torr at 20 °c? assume the mixture formsan ideal solution. the vapor pressure of benzene (c6h6) is 75 torr and the vapor pressure of toluene (c7h8)is 22 torr at 20 °c. b) what is the composition in mole fractions of the vapor above the solution in part a? how does this problem relate to the process of fractional distillation?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2


Chemistry, 22.06.2019 21:30, thompsonhomes1
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 23.06.2019 02:00, hayleebeals50
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
Do you know the correct answer?
A) whatis the composition in mole fractions of a solution of benzene and toluene that has a vapor pr...
Questions in other subjects:



Biology, 19.01.2020 07:31



Mathematics, 19.01.2020 07:31



Chemistry, 19.01.2020 07:31

Biology, 19.01.2020 07:31


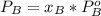
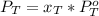
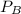 is partial pressure for benzene in the liquid
is partial pressure for benzene in the liquid 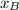 is benzene molar fraction in the liquid
is benzene molar fraction in the liquid 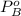 vapor pressure for pure benzene.
vapor pressure for pure benzene.