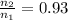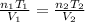Ahot-air balloon is filled with air to a volume of at 750. torr and 21°c. the air in the balloon is then heated to 58°c, causing the balloon to expand to a volume of . what is the ratio of the number of moles of air in the heated balloon to the original number of moles of air in the balloon? (hint: openings in the balloon allow air to flow in and out. thus the pressure in the balloon is always the same as that of the atmosphere.) ratio = 1

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:20, Mordred9571
Which is true of chemicals? a. things containing chemicals always cost a lot of money. b. chemicals are never dangerous. c. chemicals are in many substances in a home. d. chemicals are rarely found on earth.
Answers: 1

Chemistry, 21.06.2019 15:00, Dreambig85
Use examples from the article to explain one positive and one negative effect that chemistry has had on society
Answers: 2

Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1
Do you know the correct answer?
Ahot-air balloon is filled with air to a volume of at 750. torr and 21°c. the air in the balloon is...
Questions in other subjects:


Mathematics, 16.09.2019 17:30

Health, 16.09.2019 17:30

Social Studies, 16.09.2019 17:30

History, 16.09.2019 17:30


Computers and Technology, 16.09.2019 17:30