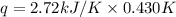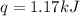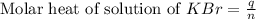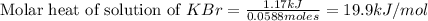Chemistry, 27.09.2019 02:30, bracefacer42
When a 7.00 g7.00 g sample of kbrkbr is dissolved in water in a calorimeter that has a total heat capacity of 2.72 kj⋅k−1,2.72 kj⋅k−1, the temperature decreases by 0.430 k.0.430 k. calculate the molar heat of solution of kbr.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:30, DragonLovely
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 01:30, nikonee
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1


Chemistry, 23.06.2019 04:10, nabeelunique
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2
Do you know the correct answer?
When a 7.00 g7.00 g sample of kbrkbr is dissolved in water in a calorimeter that has a total heat ca...
Questions in other subjects:











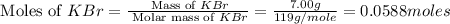
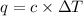
 = heat capacity =
= heat capacity = 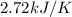
 = change in temperature = 0.430 K
= change in temperature = 0.430 K