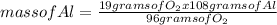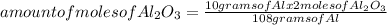Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, orlando19882000
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 02:00, rosie20052019
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1


Chemistry, 22.06.2019 10:00, zionlopez543
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1
Do you know the correct answer?
How many moles of aluminum oxide are produced according to the reaction below given that you start w...
Questions in other subjects:


Mathematics, 02.09.2021 05:30

Chemistry, 02.09.2021 05:30

Mathematics, 02.09.2021 05:30

Biology, 02.09.2021 05:30

Social Studies, 02.09.2021 05:30


Mathematics, 02.09.2021 05:30