Chemistry, 26.09.2019 21:30, dayanaraa61
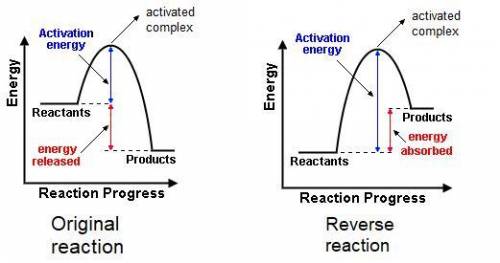
The gas phase reaction: cl(g) + hbr(g) →hcl(g) + br(g)has an overall enthalpy change of −66 kj. the activation energy for the reaction is 7 kj. a) draw the potential energy curve for the reaction and label ea, ∆e, and the activated complex or transition state. b) draw the potential curve for the reverse reaction. what is ea? what is ∆e?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, bakoeboo
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 23.06.2019 00:00, vanessacox45
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 01:30, emfranco1
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
Do you know the correct answer?
The gas phase reaction: cl(g) + hbr(g) →hcl(g) + br(g)has an overall enthalpy change of −66 kj. the...
Questions in other subjects:

Social Studies, 09.07.2019 15:20

Chemistry, 09.07.2019 15:20

Business, 09.07.2019 15:20

Business, 09.07.2019 15:20

Biology, 09.07.2019 15:20

Mathematics, 09.07.2019 15:20

Physics, 09.07.2019 15:20


Social Studies, 09.07.2019 15:20

Biology, 09.07.2019 15:20